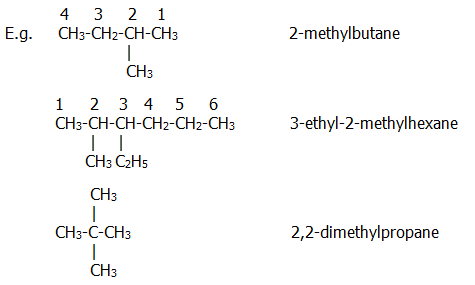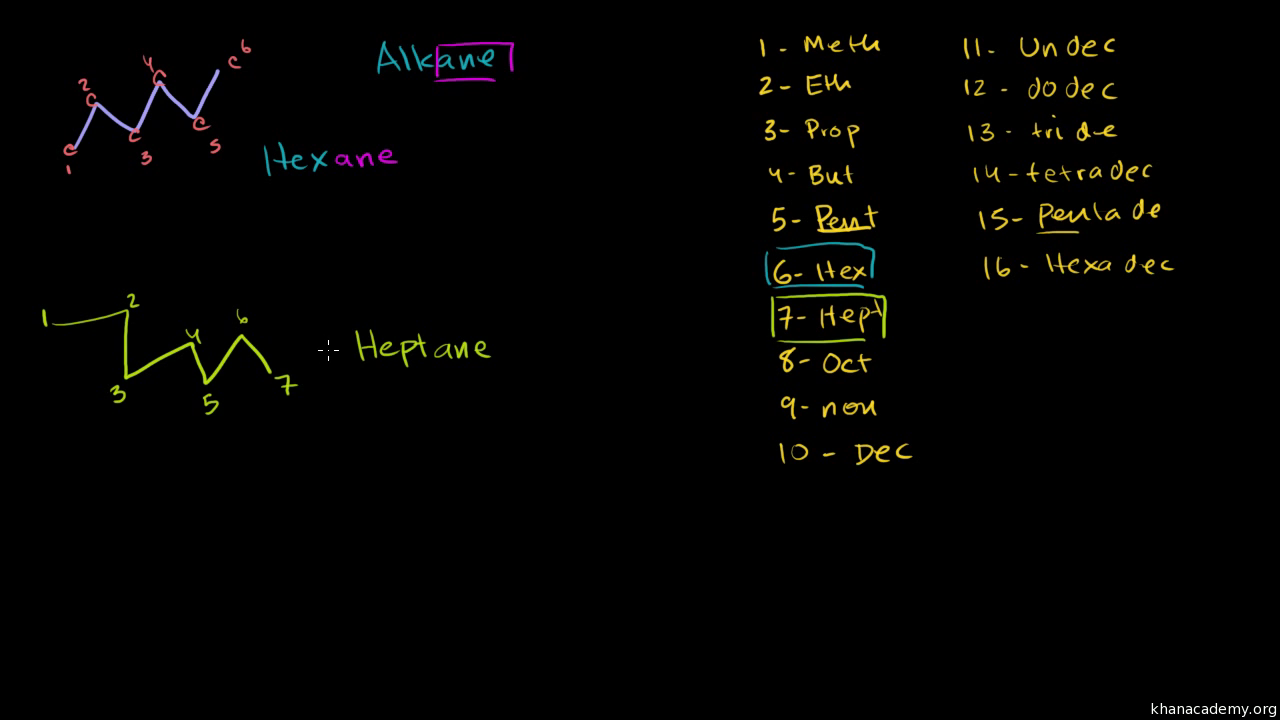Organic Chemistry Nomenclature Of Alkanes
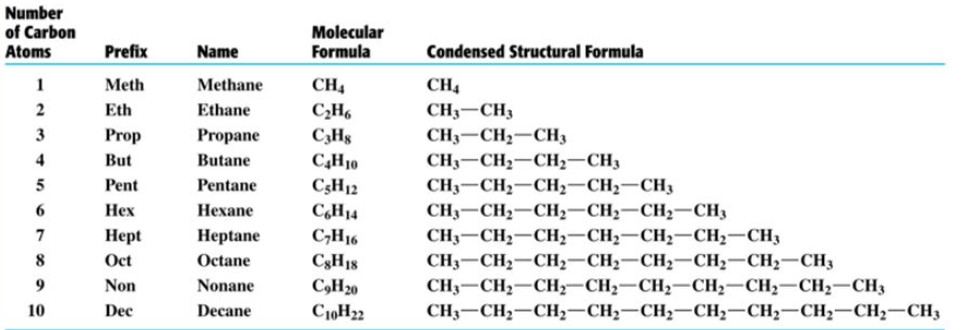
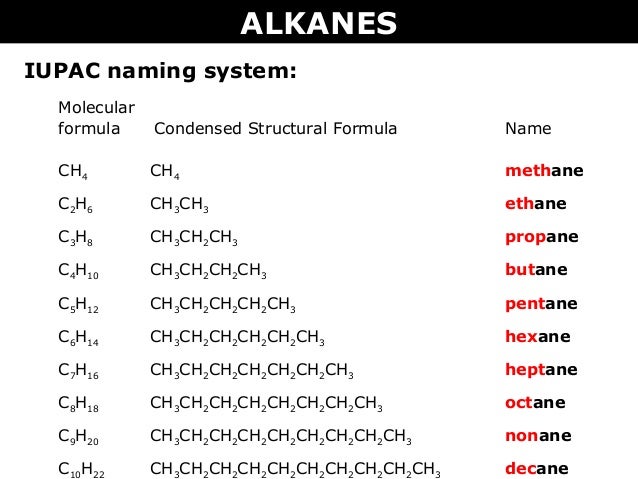
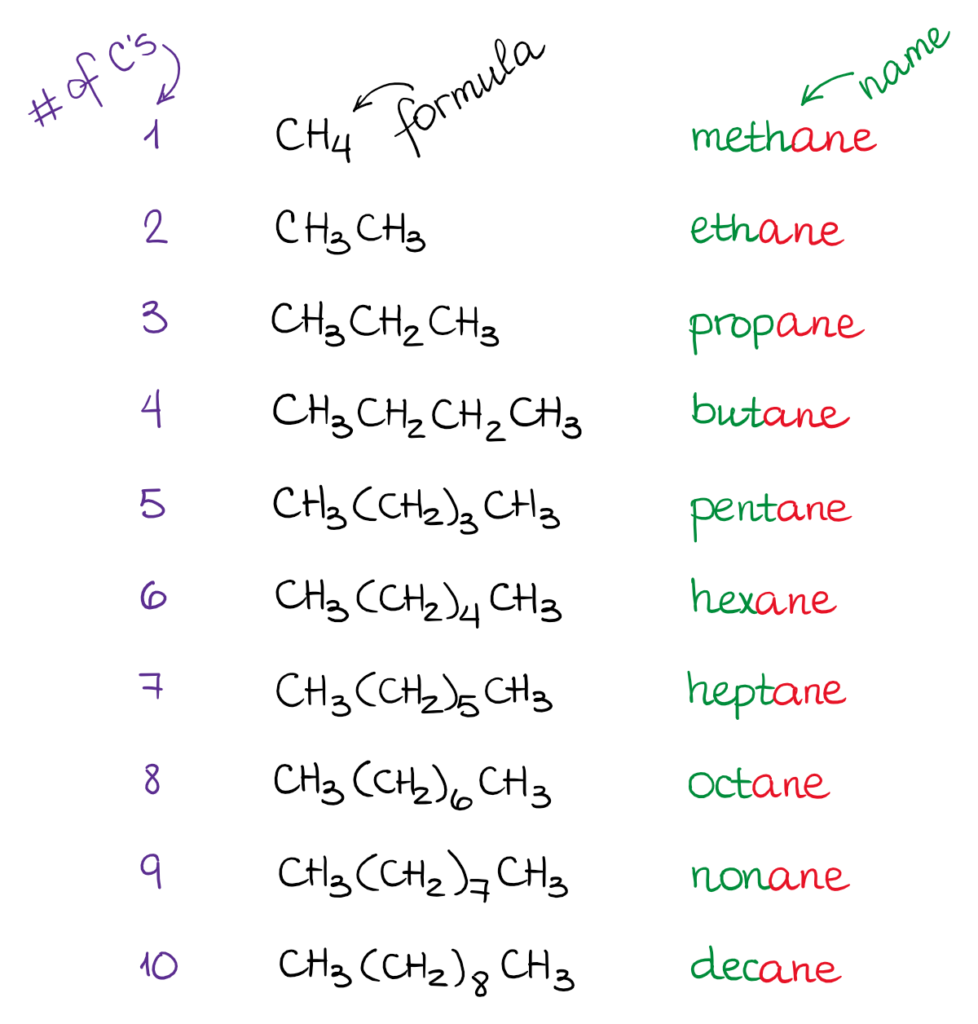
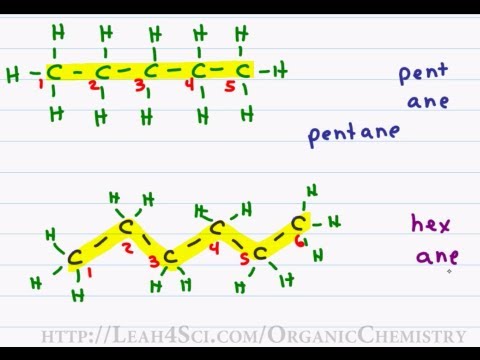
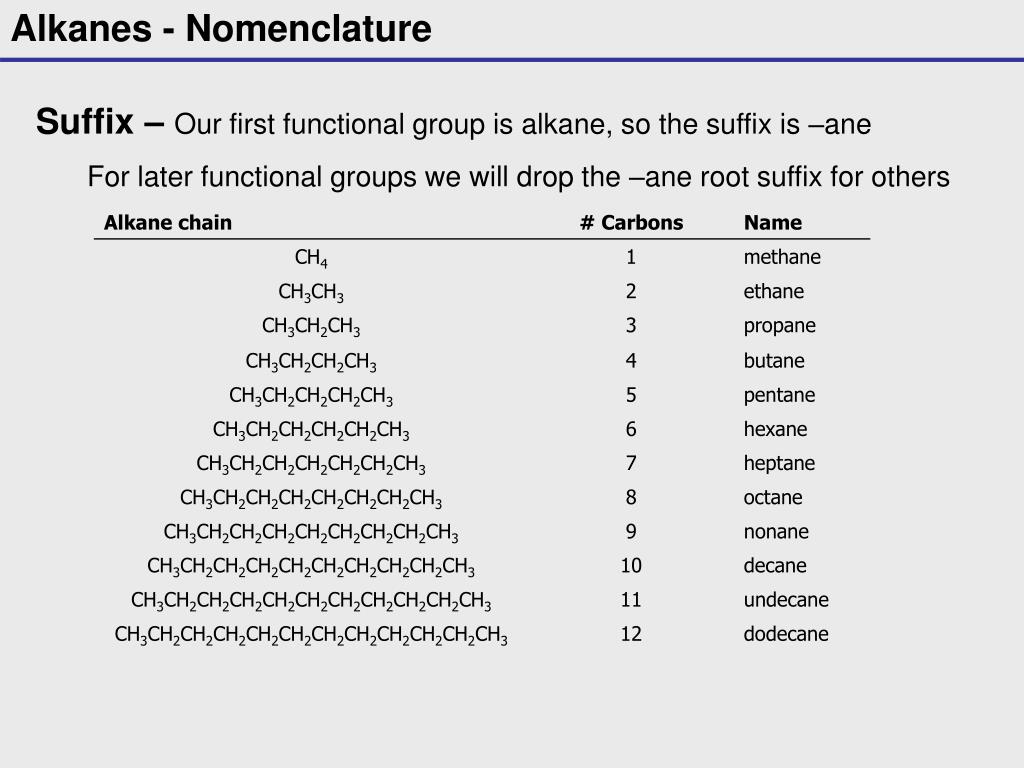
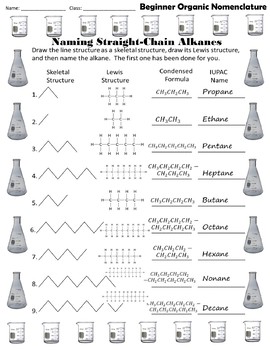
Hence c 5 h 12 is called pentane c 6 h 14 is called hexane c 7 h 16 is called heptane and so forth.
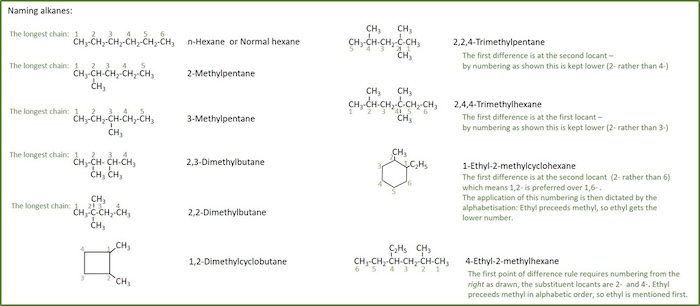
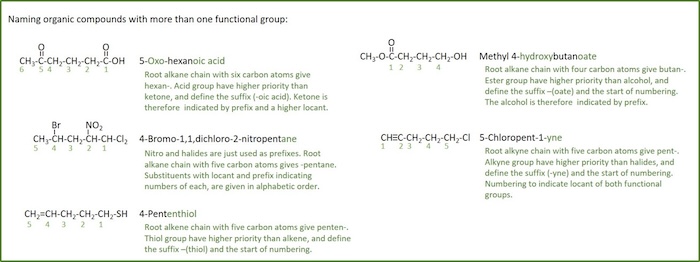
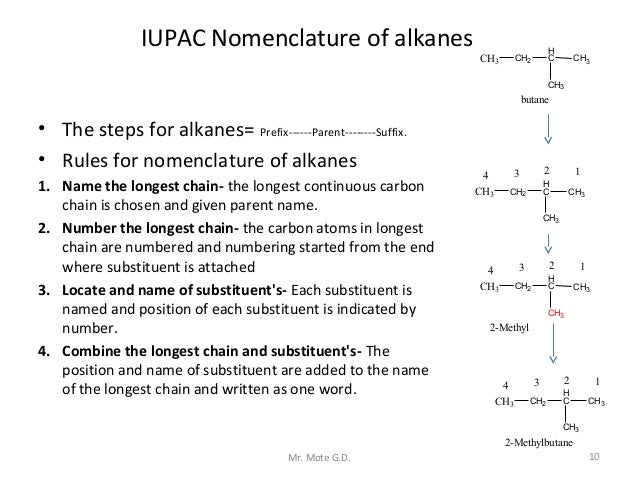
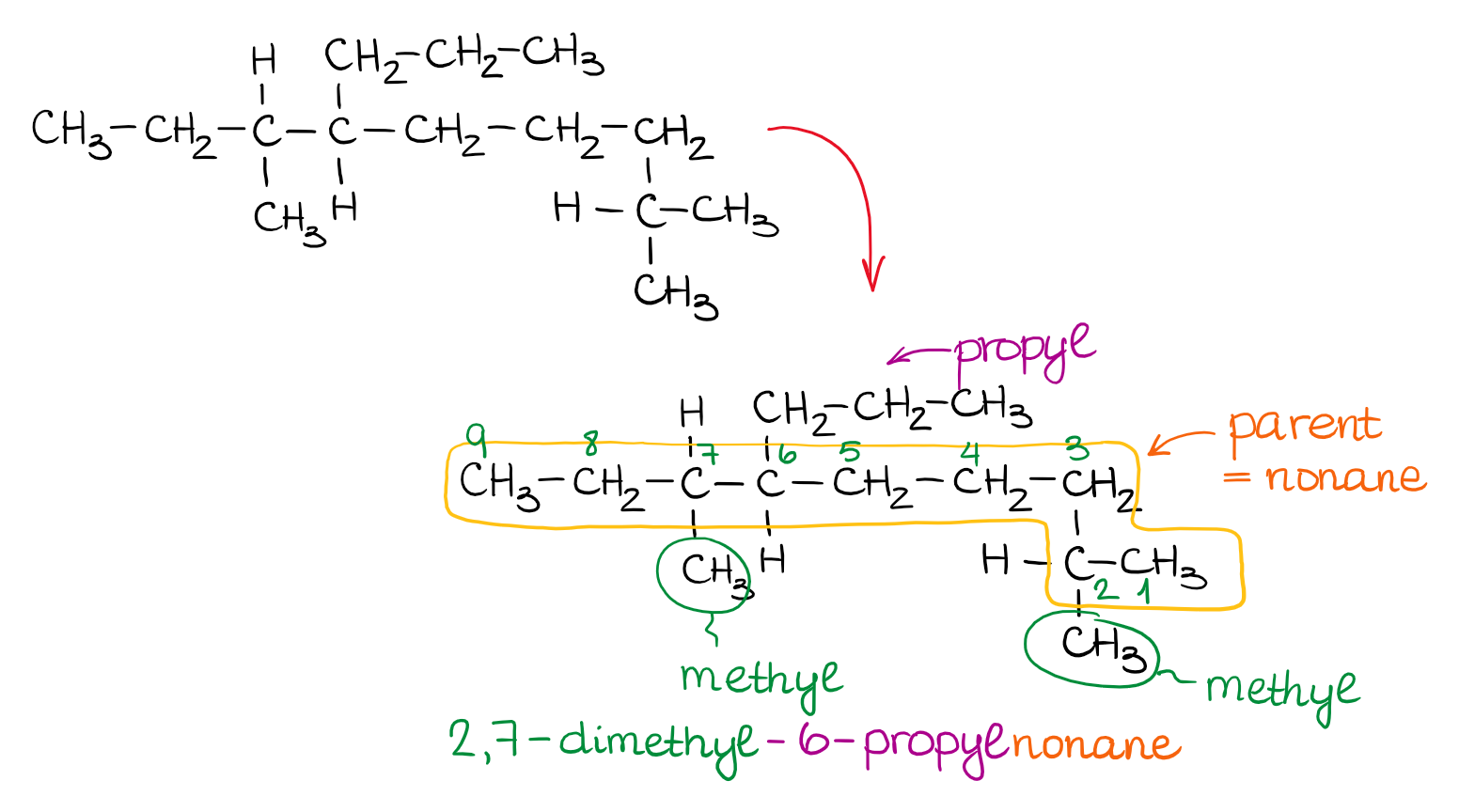
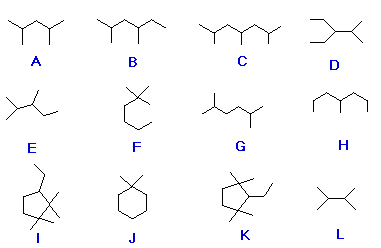
Organic chemistry nomenclature of alkanes. The following table lists the iupac names assigned to simple continuous chain alkanes from c 1 to c 10. However cis and trans are relative descriptors. Longer chain alkanes are well known and their names may be found in many reference and text books. For alkanes the following rules apply.
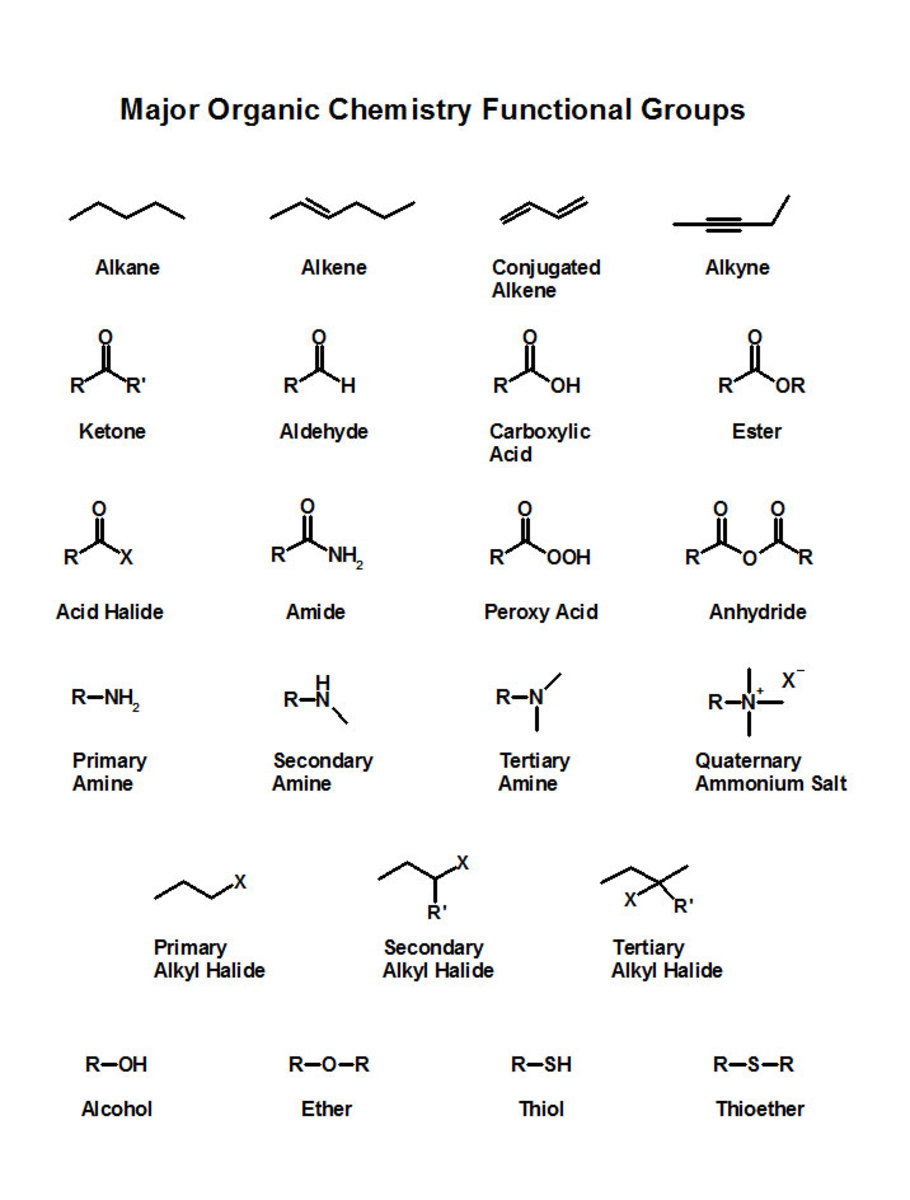
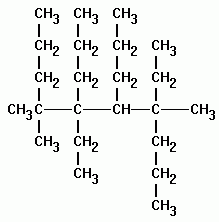
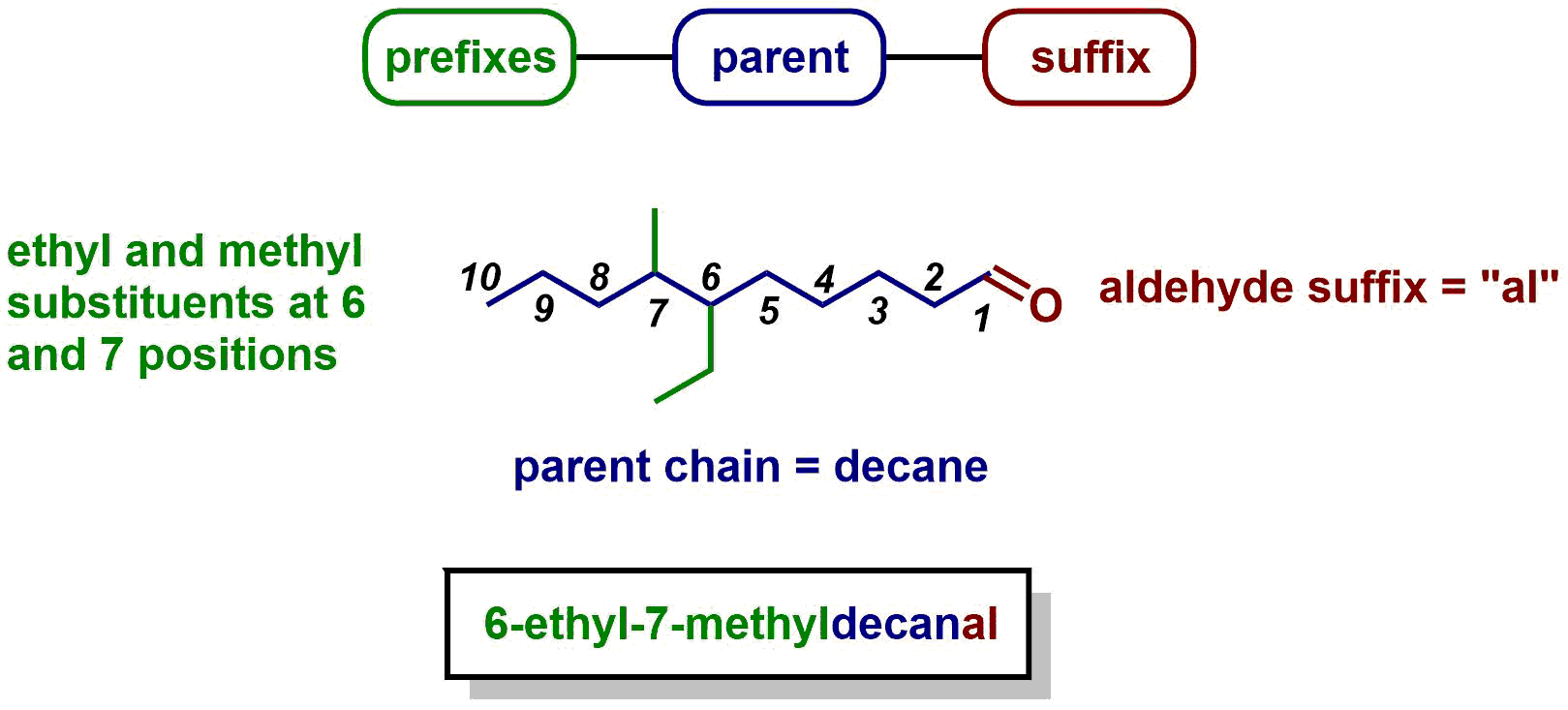
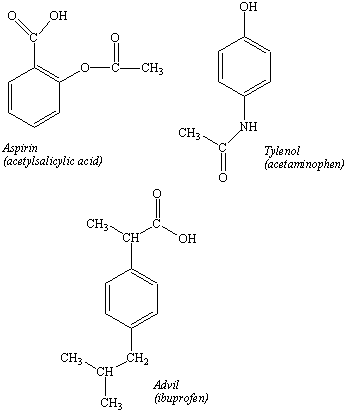
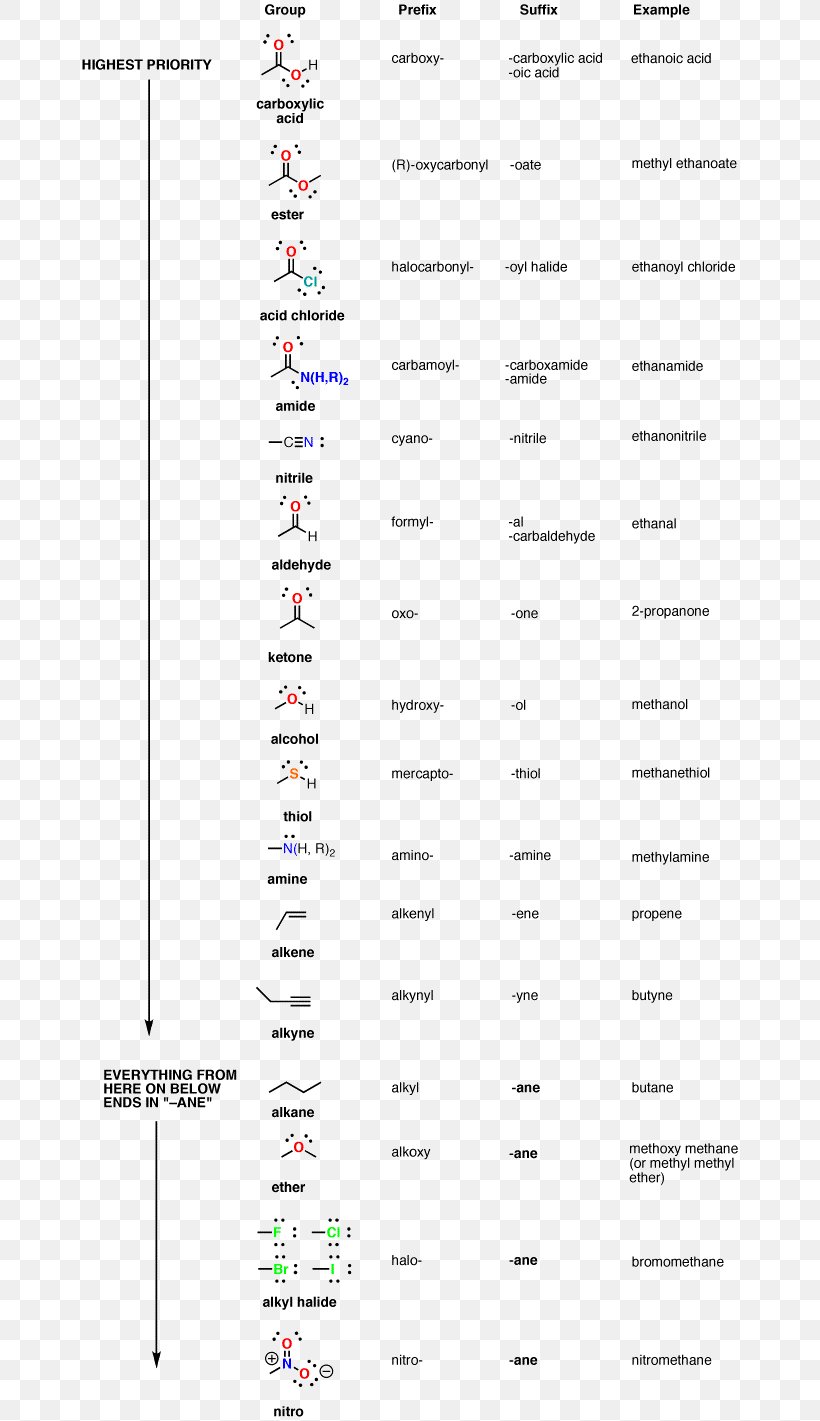
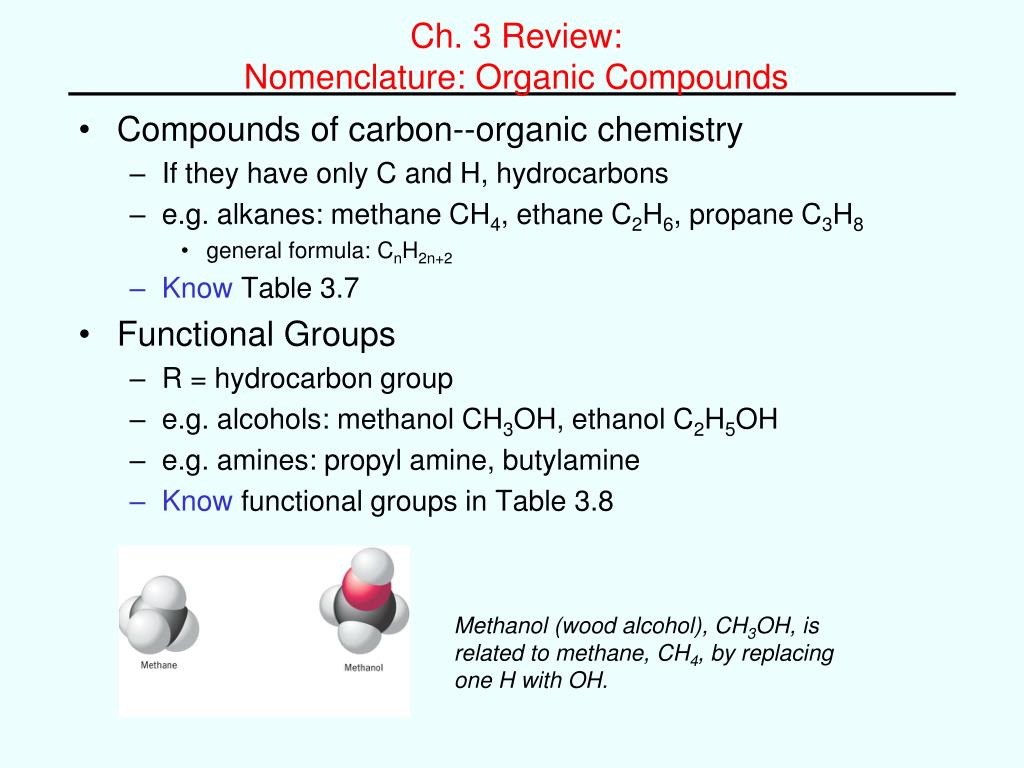
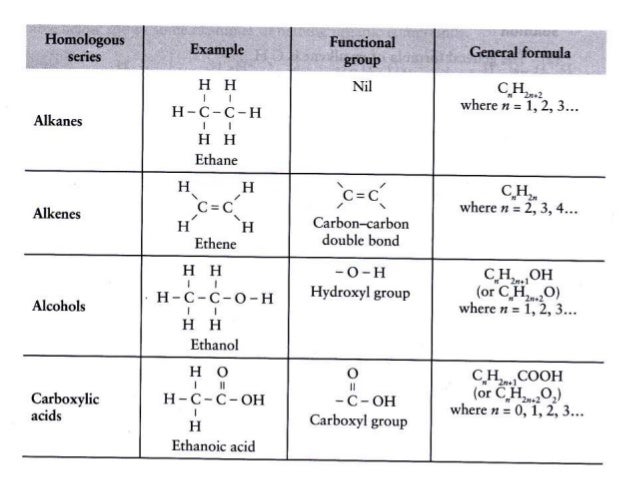
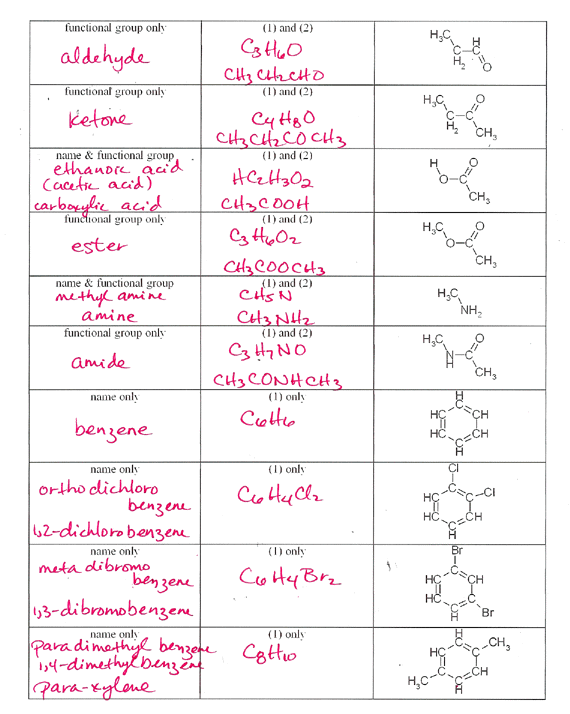
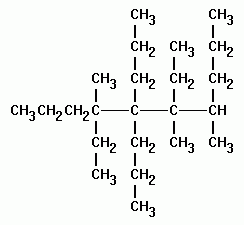
A common ane suffix identifies these compounds as alkanes. Well be learning about different aspects of molecular structure including common functional groups and conformations. The properties of organic molecules depend on the structure and knowing the names of organic compounds allow us to communicate with other chemists. In other words an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carboncarbon bonds are single.
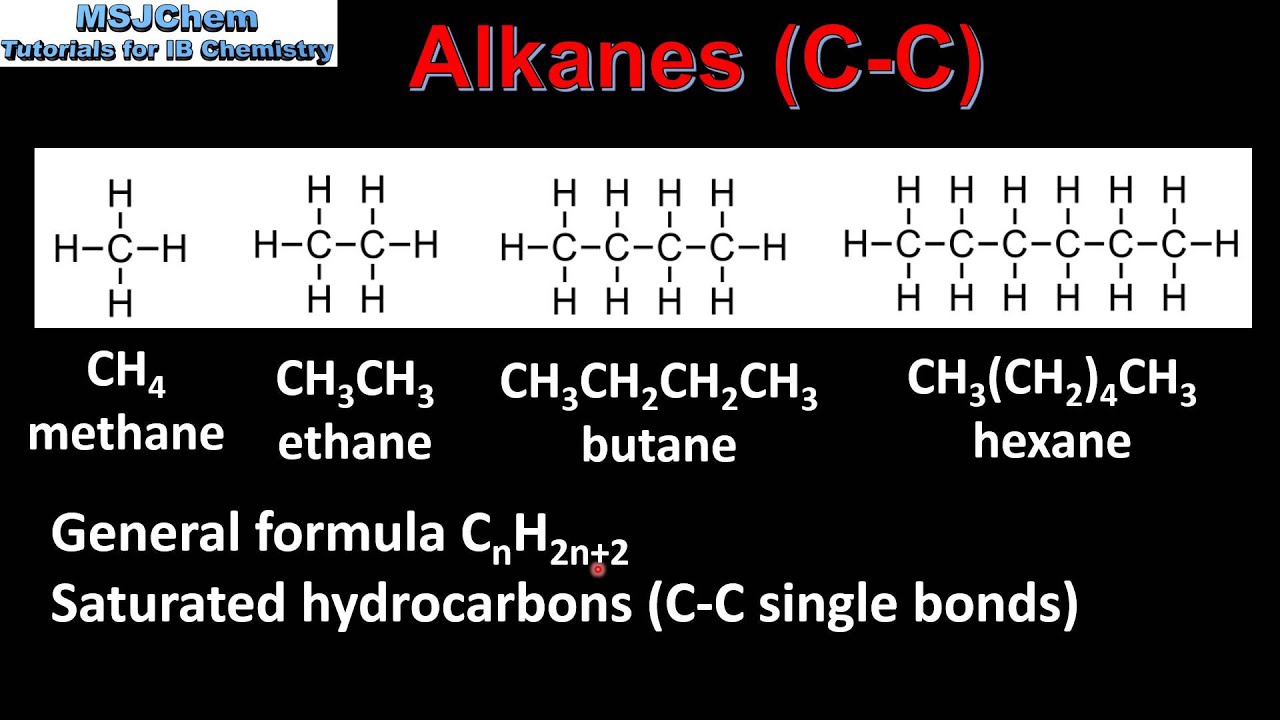
A common ane suffix identifies these compounds as alkanes. It is iupac convention to describe all alkenes using absolute descriptors of z same side and e opposite with the cahningoldprelog priority rules. Alkanes have the general formula c n h 2n2. All names of alkanes are built from the list of parent names.
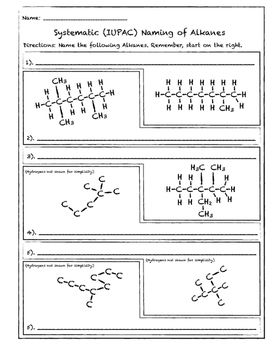
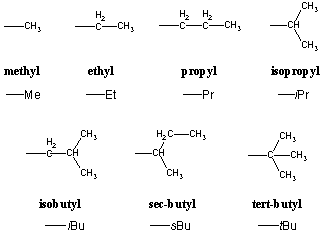
Simple cis and trans isomers may be indicated with a prefixed cis or trans. The following table lists the iupac names assigned to simple continuous chain alkanes from c 1 to c 10. Naming alkanes with ethyl groups opens a modal alkane with isopropyl. Naming alkanes is one of the first topics youre going to cover in your organic chemistry course.
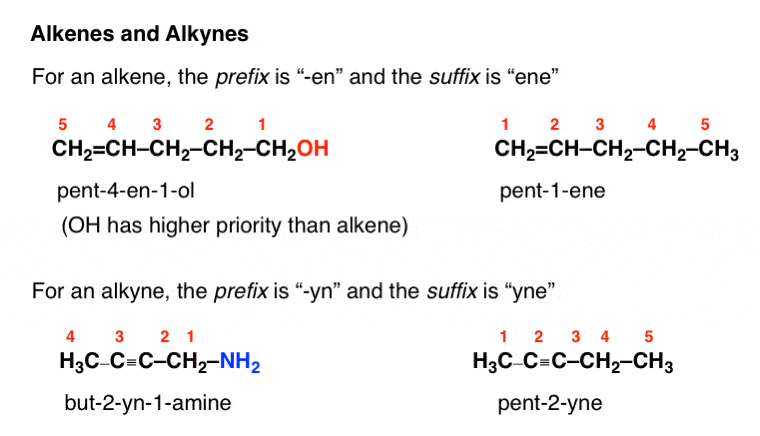
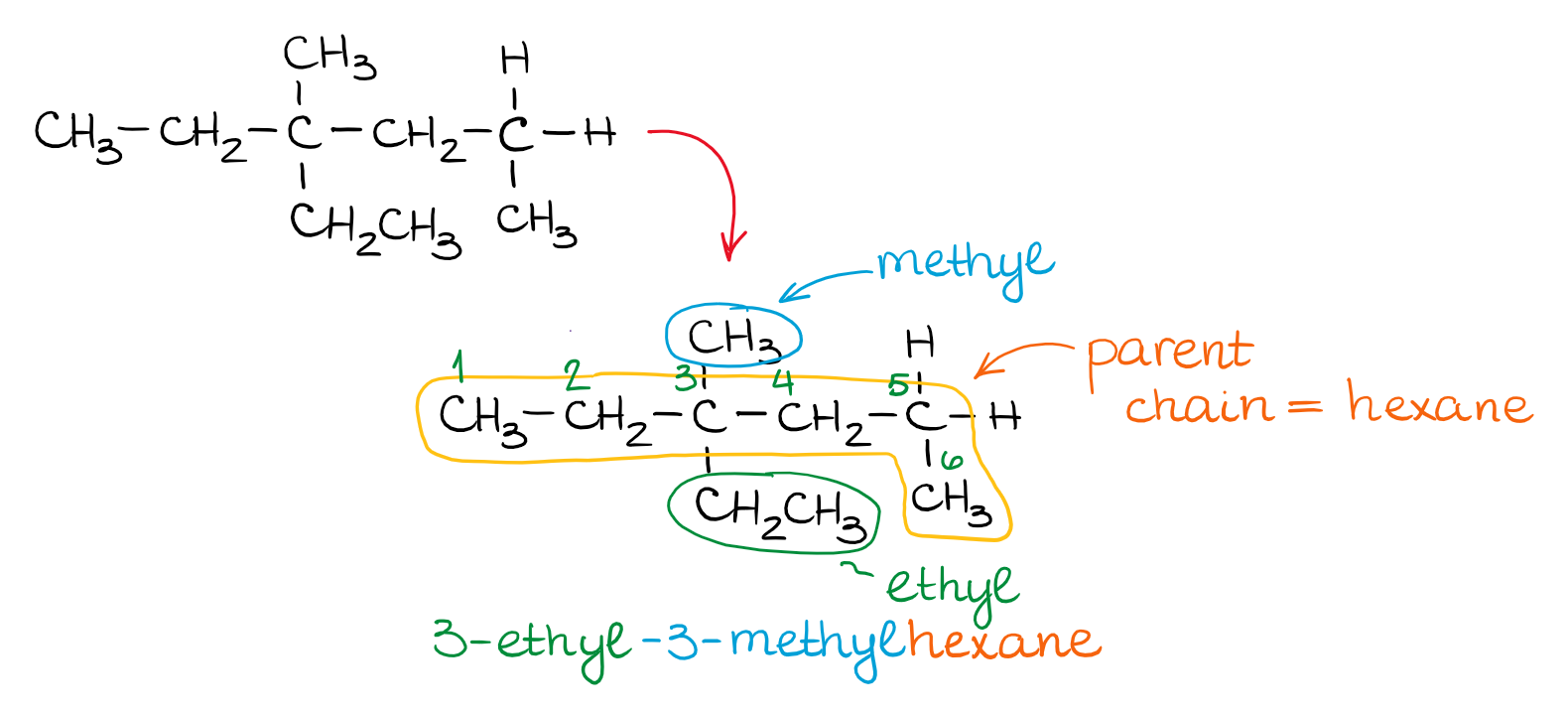
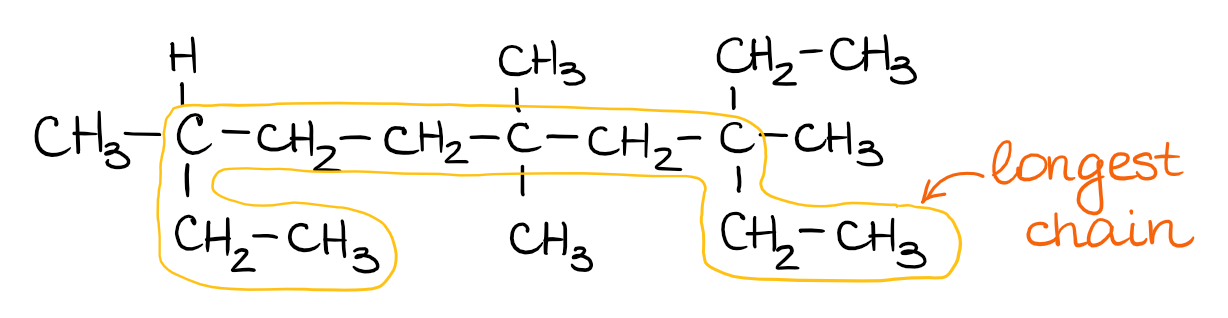
Cis but 2 ene trans but 2 ene. In general the base part of the name reflects the numberof carbons in what you have assigned to be the parent chain. Although many different types of nomenclature or naming systems were employed in the past today only the international union of pure and applied chemistry iupac nomenclature is acceptable for all scientific publications. In organic chemistry an alkane or paraffin a historical name that also has other meanings is an acyclic saturated hydrocarbon.
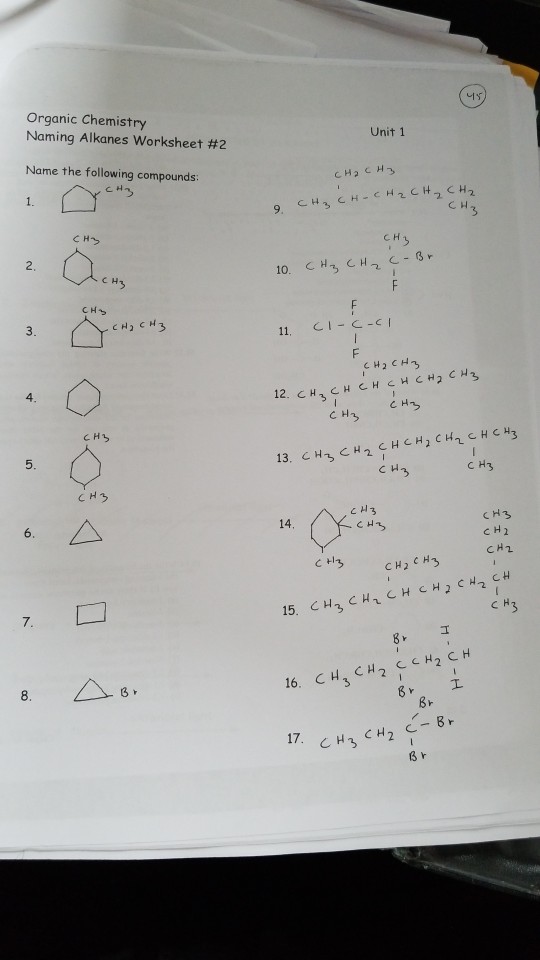
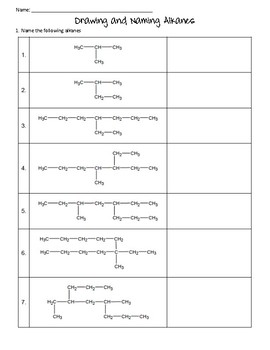
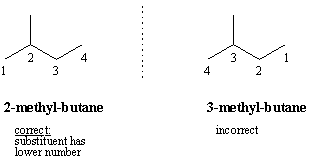
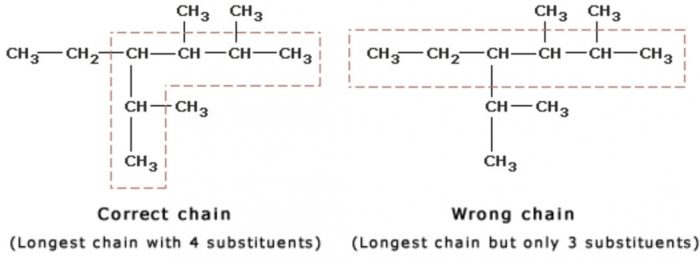
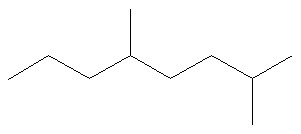
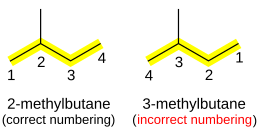
In this system a series of rules has been created that is adaptable to all classes of organic compounds. Alkanes have the general chemical formula c n h 2n2. Alkanes are the simplest organic molecules consisting solely of singly bonded carbon and hydrogen atoms. Here are some basic rules we use for the alkane nomenclature.
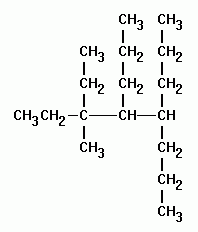
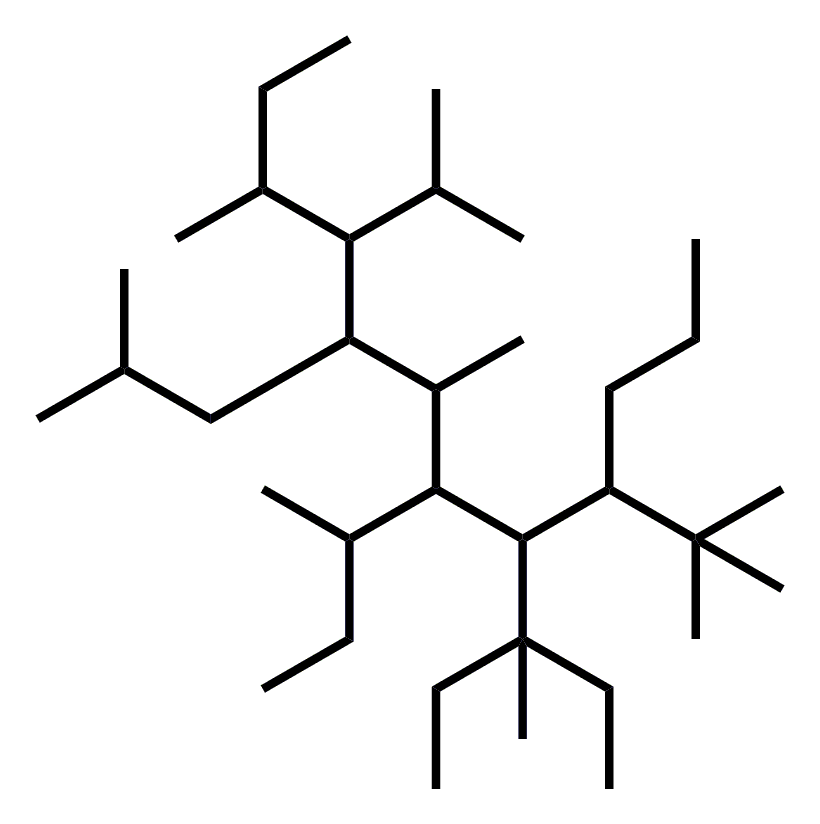
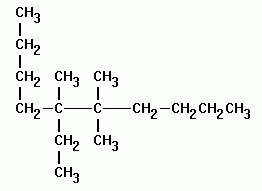
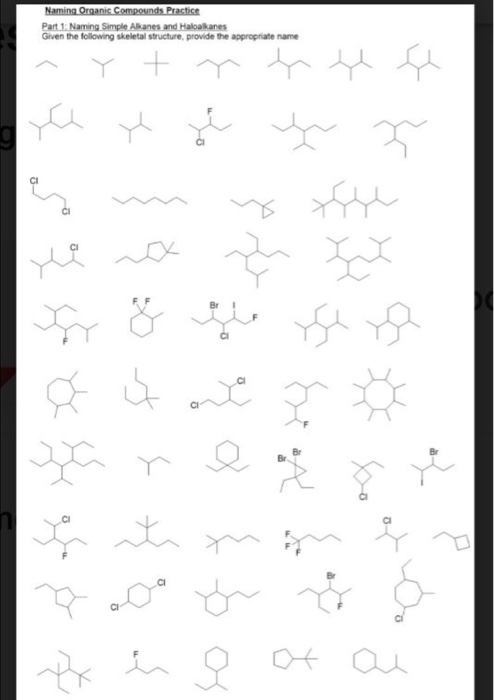
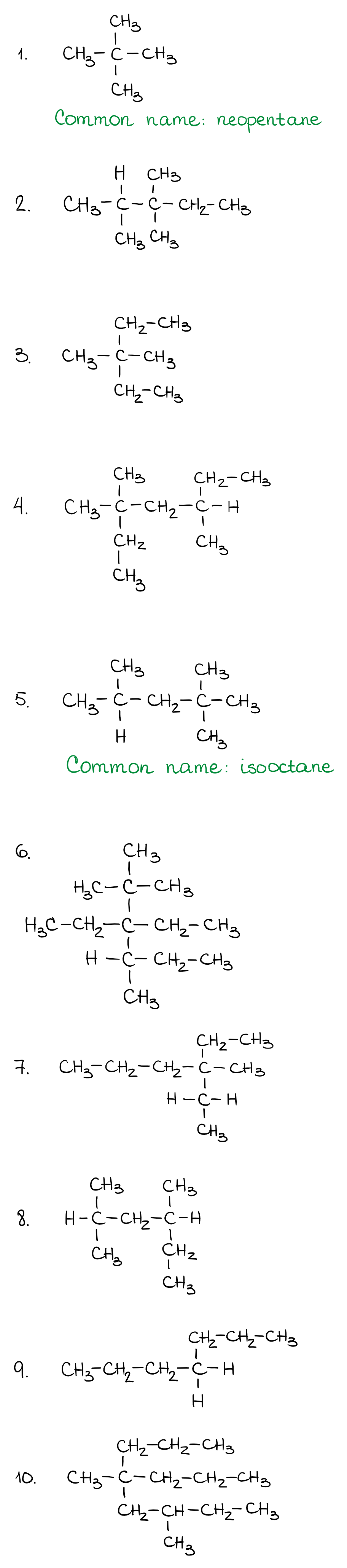
Alkanes with five or more carbon atoms are named by adding the suffix ane to the appropriate numerical multiplier except the terminal a is removed from the basic numerical term. Although their reactivities are often rather uninteresting they provide an excellent basis for understanding bonding conformation and other important concepts which can be generalized to more useful molecules. Nomenclature of alkanes is not particularly difficult but it can be tricky and tedious.